Latent Heat
Latent Heat: Overview
This topic covers concepts such as Latent Heat, Latent Heat of Fusion, Latent Heat of Vaporisation, Triple Point, Factors Affecting Evaporation, Change of State, Temperature Versus Time Graph for Heating of Ice, and Fusion of Substances.
Important Questions on Latent Heat
Does density affect boiling point?
What causes something to have a higher boiling point?
What is the most important aspect affecting the boiling point of water?
What are the factors affecting on boiling point?
What can affect Boiling point of water?
Two systems are in thermal equilibrium. The quantity which is common for them is
Select the examples of regelation from the following.
Select the examples of regelation from the following.
Liquid and solid states of a substance co-exist in thermal equilibrium during the process of:
In the temperature-time graph of heating of ice, the portions parallel to the time axis represents:
In the temperature-time graph of heating of ice, the melting point of ice is:
Temperature versus time graph for heating of ice:
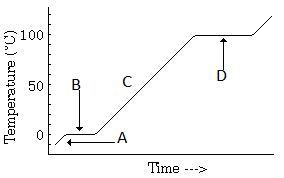
The part of the graph, which shows the thermal equilibrium of ice and water is represented by:
When the air is dry, the rate of evaporation.
The correct reason behind cooling of a room after the water has been sprinkled is
When sun rays are focused on an ice block using a lens of diameter , of ice got melted in . Calculate the heat received per minute per square centimetre. (Latent heat of ice )
The hotter the day, the ______ is the evaporation.
Sneha’s mother washed these clothes and put them to dry in the sun. Which one will take longest to dry?
It does not help in drying clothes faster.
The best method for the separation of naphthalene and benzoic acid from their mixture is
